Drawing pristine water doesn’t come as cheaply as before, and neither does cleaning it up after use. Both efforts will run up tabs measured in time, dollars, capital infrastructure expenses, and in steady “care and feeding” with chemicals.
Even as untainted water tends to be less readily available, contaminants within are multiplying in number, strength, and harmfulness. The latter fact brings to bear tougher standards for treatment, both for potability and discharging. Meanwhile, this trend comes at time of dwindling revenues for cities and towns.
On the bright side, the squeeze has also spurred innovations. Better technologies are emerging. Water departments are steadily improving their control over treatment processes. They’ve learned how to do more with less. The following discussion offers a dozen timely improvement suggestions from industry observers.
First, concerning one of the “biggies” to impact water delivery in recent years:
Arsenic Removal
It’s been a decade since EPA tightened the screws fivefold on arsenic in water, from 50 parts per billion (ppb) down to just to 10 ppb. In 2002 this move by EPA sparked lawsuits and stoked anxieties over how the nation’s thousands of water purveyors and millions of well owners could hope to pay for expensive removal technologies.
The potentially huge and lucrative market for treatment has spurred a range of innovations and solutions (see several, below). But in recent years, the challenge of how to find money has reportedly only worsened, as several commentators observe.
Jim Pardini, of the Isolux Technologies Division of MEL Chemicals, Inc. (US office, Flemington, NJ), travels the country giving informative talks on arsenic issues to water agencies and others, in preparation for offering MEL’s specific remedies. He explains, “basically all removal options are expensive.” There’s no single cheap solution. Also, sites needing remediation tend to be unique, requiring someone with expertise to size them up and devise custom treatment.
He adds that if there is a preferred low-cost alternative, it would be simply to find a new water source, and a number of water purveyors have reportedly lucked out and done this. They either go prospecting for new wells or run pipes to known low-arsenic water supplies. Drilling blindly in the water table for uncontaminated pools is somewhat akin to prospecting for precious metals.
Failing that solution, what’s next?
Pardini finds that grant money is available from both federal and state sources. Not surprisingly, though, “There’s not nearly enough for every district that needs to do something,” he says. “Not even close.”
What money there is, typically isn’t earmarked for arsenic per se, but for water infrastructure, and arsenic stands as just one of scores of competing priorities. To illustrate, Pardini notes that the State of California has published a list of about 400 recent grant applicants, “in a state where there are probably more than 1,000 water districts that need help, just with arsenic,” he says. In that numbered list of recipients, the first grant for arsenic removal ranked a lowly 49th.
This may seem to suggest that the hazards of arsenic are perceived by agencies as somewhat exaggerated. In fact, the opposite is likelier true. Pardini notes that EPA’s early assessment is busy being affirmed and documented. For example: in Bangladesh and India, serious arsenic poisoning from drinking well water is on the increase.
What actually dampens the sense of an “arsenic crisis,” though, is the fact that its toxicity “works by slow accumulation and does not produce symptoms overnight” (unlike sewage contamination, for instance). On this point, the State of New Jersey, for one, has declared EPA’s 10-ppb standard not tough enough. That state now caps allowable limits at just 5 ppb.
One of the firms that sprang up in 2002 to offer solutions to this vast and instantaneous market was AdEdge Technologies, of Buford, GA; they have since discovered a niche of smaller communities and well sites not being aggressively pursued by the dominant treatment providers, notes the company’s Joe Naylor.
To address the funding problem, AdEdge recently formed a subsidiary company able to connect needy water districts and others to willing lenders–of which there is apparently no shortage, Naylor reports.
Chlorine Testing and Removal
The foregoing hardly completes the discussion on arsenic, but moving on to another, sometimes pesky, presence: the removal of a toxin that was first deliberately added.
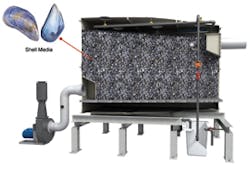
The “Monashell” filters odors and volatile organic compounds (vocs) from wastewater pipes, using calcium-rich seashells.
Chlorine goes in to kill bacteria, of course, but chlorinated water must be stripped of this before being potable or dischargeable. First, the selection of specific chemicals for chlorinating makes a difference in cost, notes Lou Finelli, a regional sales manager for Lonza Microbial Control, Water Division (known as Arch Chemicals, Inc., acquired by Lonza in 2011). The vast majority of water treatment agencies are probably using cheap liquid sodium hypochlorite (i.e., bleach) for this, but, he suggests, there is perhaps unperceived waste and loss here–due to its rapid degrading. Ultimately, this results in spending too much on bleach, then on over-feeding the water.
Far preferable might be a switch to calcium hypochlorite, which, has a “very, very long shelf life–a year and a half in the bucket. It’s a better choice for treatment plants,” he says, especially those that do not use heavy volumes.
Even more critical, though, is the issue of precision, both in application and in dechlorination. The more perfectly measured the chlorine injection, the easier and cheaper comes it extraction.
Here, Finelli finds that water treatment agencies are sometimes guilty of using outmoded or simplistic sampling methods for measuring the chlorine residue. It is not uncommon, for example, for treatment agencies to use a basic DPD (N,N Diethyl-1,4 Phenylenediamine Sulfate) colormetric or litmus test, or pH kit, to gauge whether chemistry has stabilized in its proper range. While these tools are fine for swimming pools and the like, “they’re not a real definitive way” of measuring chemicals, he says.
Far preferable is the installation of a sensitive in-line instrument, which measures chemistry “down to two or three parts-per-million or milligrams,” such as by gauging the water’s electrical conductivity. Integrated data logging and reporting also makes the plant operator’s job easier, he adds.
Along with doing precise measurements comes automation of chemical addition. This minimizes possible over- or under-feeding. Doing loose approximations is often wasteful and sometimes dangerous, Finelli says.
For good results, use positive displacement pumps for chemical feeding, he recommends; these “are very precise metering machines which allow exact amounts to be fed at any given time.” Yet, he finds that many water treatment agencies seem to lack them at key points.
An adequate control system is defined as one assuring not only correct volumes or ratios, but proper chemical contact time. Also on the theme of chemical monitoring and feeding, John Becker, president of Analytical Technologies (ATI) recommends a system for controlling sodium bisulfite used for dechlorination of wastewater, “which is one of these issues where a lot of people waste a lot of money because there’s no good way to control it,” he says.
Becker notes that, typically, residual chlorine must be reduced to meet a standard of less than 50 ppb. Because this figure is difficult to reach precisely, treatment agencies are tempted to err on the side of caution by over-feeding the dechlorination agent, usually either sulfur dioxide gas, sodium sulfite, or sodium metabisulfite.
How much money can be saved through careful readings? Becker offers this example: One million gallons of water chlorinated to 1 part per million (ppm) contains 8.34 pounds of excess chlorine. To remove 1 ppm takes 7.59 pounds of sulfite (SO2).
Also, maintaining an extra 1 ppm of sulfite residual above what is needed requires an additional 7.59 pounds of SO2.
To illustrate, he says, take the most popular chemical, sodium bisulfite. This “normally runs around 80¢ per pound of SO2.”
However, the actual cost of dechlorinating agents varies considerably by locality and type: for example, sulfur dioxide gas is usually cheapest, while sodium metabisulfite is most expensive. The price of a pound of SO2 may vary from about 40¢ to $1.00 per pound, depending on the market.
Here, then, is the general formula on savings:
Dollars saved = Cost (SO2/pound) x Flow (mgd) x 7.59 pound/ppm x 365 days/year
So, in this illustration, a 10-million-gallon-per-day (mgd) plant using sodium bisulfite costing 82¢ per pound of SO2 in a year would save nearly $23,000.
Next, consider two relatively benign elements:
Iron and Manganese
Though these don’t pose a serious health risk, they do sometimes discolor water and form corrosive deposits on things like cooling towers. So, removal is necessary. For about 50 years, the standard methodology consisted of gravity or pressurized filtration, using naturally occurring manganese green sand.
About a half-dozen years ago, a firm called Inversand, of Clayton, NJ, and a subsidiary of Hungerford & Taylor (H&T), patented a modified version, “Greensand Plus” (GSP). H&T vice president Ken Sayell offers a comparison of the old and new sands, and explains the latter’s improvement.
Depending on the particular local water’s chemical makeup, he begins, manganese green sand “always required the use of potassium permanganate to keep the media regenerated.”
But, the key gain in GSP is that it works with just chlorine as the regenerant “and does not need potassium permanganate.” Chlorine costs about one-fifth the price of potassium permanganate. That’s the big saving, “and,” he adds, “you can eliminate all the hardware–the chemical feed pumps, storage tanks, and material handling equipment for permanganese systems.”
New treatment plants are now increasingly designed to use GSP and save on this hardware and maintenance, he says. Older sites can easily switch to GSP as well. “The physical properties are identical. You don’t have to change your backwash or service rates. You can take manganese greensand out and put Greensand Plus in–without making any changes.”
Advanced Filtration Versus Chemistry
Also under the heading of plant design and equipment comes the question of relative savings attainable with advanced ultrafiltration (UF) and reverse osmosis (RO) systems. With ever-improving capabilities, these tech solutions are poised to become out-and-out permanent alternative to some chemical applications.
GE’s Paul Schuler, who is the company’s leader for new, engineered water treatment systems in North America, outlines several intriguing recent developments.
One of the most cost-effective strategies, he says, is to supplement whatever plant filtration currently occurs in existing tanks, by applying submerged membrane retrofits. Rather simply and easily, these “expand a plant or take it from the present 10 million gpd, to 20 million gpd,” an increase often necessitated by growth or the need to replace old infrastructure.
Such retrofits have been done in dozens of places so far, but there are probably thousands of plants that are candidates for it, he says, driven by the now-universal EPA requirement of filtration of surface water prior to delivery.
As for wastewater, advancements in membrane bioreactor (MBR) performance makes them increasingly candidates to replace the use of secondary clarifiers to separate treatment solids from water. By applying UF technology–“which is the pore size of your skin,” he points out–“you can create reuse water” ready for sending forth in irrigation. And in many parts of the world that water is already considered potable, or nearly so.
In this MBR or UF scenario, there’s also a significant chemical saving in treatment. Schuler explains that, “If you need to coagulate and settle a water before it is filtered, your two options are either sand filtration or membrane filtration”, and the latter, due to its much finer porosity, allows saving on, or substituting, chemicals for flocculation and sedimentation.
And a second breakthrough with MBR: there are now several treatment plants in North America that are actually turning off UV disinfection at this stage. How’s that possible? Schuler explains: “The filtration size is now so small that it filters out all the bacteria that we would normally disinfect.” A conventional treatment plant needs four steps: primary clarification, biological treatment, secondary clarification, and then disinfection. But in this new scenario, “You’re going to meet all the permit criteria with the membrane filtration, because you’re filtering out everything.”
To date, states such as Missouri and Michigan have accepted the concept of dispensing with UV (a costly and energy-intensive step) when unnecessary, and many more state approvals are anticipated, notes Schuler.
Lastly, he says, due to such factors as improved scalability, sizing of membrane cassettes, and ease of operation of MBR plants through automation, they’re viable for larger municipal treatment roles. Previously, MBR suffered from a perception that their complexity made them practical only for smaller applications.
Fully Chemical-Free Treatment
The following method uses a wholly natural remedy for wastewater treatment, applying no chemicals at all. In 1993 the developers of this “green” approach arrived in the US, coming, appropriately enough, from the “Emerald Isle”, and the firm’s branch here is based in the aptly named city of Greensboro, NC.
Tom Smith is the director of US operations and marketing for developer Bord Na Móna Plc, of Newbridge, Ireland. As he explains, Bord Na Móna (BNM), being Ireland’s national energy company, for years had harvested peat from that nation’s extensive bogs, turning it into fuel briquettes.
A few decades ago BNM discovered that peat as a cleansing resource–in combination with spent seashells, also found in copious supply on Ireland’s coasts–makes an excellent natural biofilter for wastewater plants. The effluent easily qualifies as reuse water. Moreover, shells of clams, oysters, and mussels contain high concentrations of calcium carbonate–a substance that naturally controls waste odors and volatile organic compounds (VOCs). BNM’s peat biofilters evolved into its Puraflo product, and the seashells became Compact Monashell.
Results-wise, BNM makes the remarkable claim that a much cleaner effluent results than occurs with conventional treatment–so clean that, with UV disinfection it qualifies as potable, for some overseas standards.
Treatment plant sizing with Puraflo is limited to a very small range, though, from single-family size up to a few thousand homes. Smith reports that it succeeds with intermittent and variable flows, and has a projected low-maintenance lifespan of about 15 years.
In support of BNM claims, assorted case studies are offered. One example is the nearly 400-acre Minooka Park RV campground in Chilton County, AL. Originally built with a
Chemical feeder
conventional 10,000-gallon septic tank, the system required its contents to be emptied every two months and carted off to the Calera treatment facility. Eventually the municipal plant refused to provide this service, forcing the park to seek an alternative. Campers became happy again, by picking BNM’s Puraflo. To fund installations, they won grants from Alabama and EPA.
Results are described by Glenn Littleton, who is facilitator of the Cahaba Clean Water Partnership and coordinator of the Buxahatchee Creek Watershed Restoration Project. He reports wastewater clarification at 90%, compared to the 40% typical of septic tanks. Treated effluent runs to a leach field. Unlike water treated at Calera, the BNM Puraflo requires almost no energy.
For its part, Monashell can be installed by wastewater agencies to remove hydrogen sulfide odors and VOCs within hours.
Dollar-wise, says Smith, “They’re all very cost effective because the system uses no chemicals or carbon, and very little energy.” Payback periods will vary, but the company claims norms ranging from one to five years.
Final Thoughts
GE’s Shuler offers a take on these developments that pegs the driving force for all these innovations–especially those now flourishing abroad–on a new reality.
“From global perspective,” he says, “North America’s water treatment standards and practices have been shaped by our abundant water and cheap energy. But we are spoiled–and that abundance is not going to continue for the remainder of our lifetimes. We need to look to places where water quality and quantity are limited and the cost of energy is high–like Asia or Africa. New technologies are being accepted there, and those places are bringing practices, work processes, and technologies to North America to make us better.”
One dynamic and positive impact of EPA’s ruling has been to spark a proliferation of innovative methods and technologies, all vying for this potentially huge and lucrative sector.AdEdge’s system perhaps typifies many. The company’s Joe Naylor explains it, adding that “granular ferric oxide or manganese dioxide chemicals to inflowing water” begins the process of removing its heavy metal toxins, “either through coagulation, filtration, oxidation, or adsorption.”
In the case of AdEdge, another proprietary process called H2Zero comes along to save water by recycling the backwash; after the first usage, it flows to a holding tank where particles settle. The clarified water is then pumped back for reuse.
Jim Pardini, of the Isolux Technologies Division of MEL Chemicals, Inc., describes Isolux’s solution by noting that it falls under the major class of adsorption systems, “and there are a lot of small, remote sites for which adsorption will be the best alternative.” Among the rival adsorber media, headed by iron-based ones, Isolux’s is unique for using zirconium hydroxide. MEL, he points out, is the world’s largest producer of zirconium chemicals.
Siemens Water Treatment, a dominant provider globally, answers the arsenic challenge with granular ferric hydroxide (GFH) media. In the course of deleting the target poison, it also adsorbs unwanted selenium, phosphate, chromium, antimony, and copper.
The process is described by Gerry Baker, a Siemens application engineer: Iron already in most water becomes oxidized, and in most cases does not require preoxidation; arsenic can then be adsorbed, filtered out, and disposed of. In cases where the iron content is insufficient, says Baker, “you’d need to supplement, with chemicals, for it.”
Process-wise, though, backwash is needed every two to six weeks to prevent compaction and remove captured contaminates. This infrequent rate yields low volumes of wastewater and filtrate: hence, water is being conserved. Baker explains: “The whole idea with GFH is that if it’s used properly you have reduced cost because of less backwash frequency and less wastestream.”
The Importance of Plant Design
Simply changing chemicals without altering structure underscores another topic to consider, regarding capital cost-saving opportunities in plant designs or redesigns. This often results when decisions are driven by well-integrated, gentle, and efficient chemical treatment strategies. An added benefit is the operational savings. This applies across the spectrum of water and wastewater issues.
Harold Aronovitch, who is an H&T vice president and its technical director, advises many consulting engineers and water agencies on plant designs. On this he points out that while selection and application of chemicals will either save on, or add to, overall costs, the primary driver is always the water itself that’s being treated, and local water conditions always seem to dictate unique analysis.
Within this mandate, then, various cost-saving alternatives can usually be weighed and compared. An interplay between material cost, labor, time, and consequences often results. Aronovitch illustrates:
“Suppose water requires [pH] neutralization, and someone asks, “˜Should we use sodium hydroxide or potassium hydroxide?’ The first thing I would say is that potassium hydroxide costs more. I would then ask, “˜Do you have an issue with sodium in your water so that you’d have to use something that would be more expensive? Otherwise, use sodium hydroxide.’ “
Another example: “Potassium permanganate comes in dry crystals, which you have to dissolve,” he says. “It has limited solubility so that you have to have a mix tank. And you have to add limited amounts. If you add too much, it will start dropping out on you and you end up with solids on the bottom of your tank.
“As an alternative, and more expensive, of course, you buy something like sodium permanganate, which is also an oxidant,” he adds. “Buy it in 20% or 40% concentration–and that would cut down on the labor involved. But you’d have a higher…operating cost, buying that chemical.”
Aronovitch continues: “Rather than using potassium permanganate or sodium permanganate, if you could end up using bleach–sodium hypochlorite–that would be even less expensive.”
In one recent design conference with consulting engineers, he recalls, the decision was made to go with costlier potassium permanganate over chlorine in a new municipal plant, “because the client had been using it in the past–and they have an older workforce. Rather than try and pull something new on them, they decided to stick with something that everyone already knew. Also, they were a fairly small utility, and the cost saving would not amount to that much of a difference.”
This anecdote illustrates the obvious real-world fact that chemical choices can’t always be reduced to pure cost factors; human resource issues sometimes trump costs. Likewise, political, perceptual, and regulatory forces come to bear, and not infrequently, proprietary commercial patents or trade secrets also impose constraints.
In another illustrative case, a water plant opted to use hydrochloric acid as a regenerative medium, as opposed to cheaper sulfuric acid. The sole reason was that the water agency balked when contemplating a potential public relations nightmare if it was perceived to be routinely transporting truckloads of sulfuric acid through town.
Another real-world factor is that, due to sometime complex local variables in water conditions, and the steady advent of product innovations, there’s often no true expert consensus on what is “best available practice.” Hence, a need for experimentation and testing arises.
Example: One engineer came along with a design proposal “for putting in a clarifier and filters in order to treat a water which had hardness and lots of iron,” for a textile plant. Aronovitch suggested instead an ion exchange process using acid regeneration. It was a method that, he says, “to my knowledge had never been done, with that much iron in the water.” When it succeeded, the eventual savings easily justified the expense of the pilot test.
Lastly, Aronovitch notes, steadily rising costs of wastewater disposal tend to alter economics over time. What was right a decade ago may no longer be. In such cases, he says, “Any water that [agencies] could reuse or cut down on, using an alternative process, could bring tremendous saving.” He sums up, “The tools are already there. It’s how you apply them that can be innovative.”